 Phenol is a toxic organic chemical found in many foods and chemicals in our environment. Some people have an allergic reaction to phenol.
Phenol is a toxic organic chemical found in many foods and chemicals in our environment. Some people have an allergic reaction to phenol.
Because phenol is found in so many chemicals, products and foods, you are likely to have quite a high exposure to it at times. This depends on your lifestyle, job and another of other factors.
Phenol is also known as carbolic acid and hydrobenzene. It is most commonly produced from coal by distilling coal tar or the partial oxidation of benzene.
It is used as a preservative in some allergy serums. If you have certain types of allergy tests, your reaction to phenol must be checked as part of the test. If you react to phenol, this must be taken into account when doing the rest of the tests.
It is found in a diverse range of products including artificial colours, artificial flavors, BHT/BHT and natural salicylates.
Phenol Uses
Phenol is a very useful chemical and has many uses.
Condensation with acetone gives bisphenol-A, a key building block for polycarbonates.
Condensation with formaldehyde gives phenolic resins, including the well-known Bakelite.
It is used in the manufacture of the following:
- Epoxy
- Aspirin and other drugs
- Picric acid explosives
- Herbicides and pesticides (constituent)
- Phenolic resin including Bakelite formed by reacting phenol with formaldehyde.
- Nylon
- Synthetic detergents
- Polyurethane
- Perfume
- Gasoline additives
- Dyes
- Cosmetics
- Sunscreen
- Hair dyes
- Skin lightening preparationsPhotography solutions
- Preservatives in medications including allergy shots
Note that while some of the above products contain phenol that may leak from the product, others may have the chemical locked in and therefore inert (meaning it won’t affect you) and still others may use phenol in production but leave none of the chemical in the final product.
Phenol Medical Applications
Phenol Natural Sources
Phenols can occur naturally. Here are some examples.
Phenol is a toxic agent in poison ivy and poison oak.
It is found in thyme oil. Thyme oil is used to produce menthol.
Spring water may contain phenol for two reasons. If the water comes in contact with naturally occurring coal, phenol may leach out of the coal and into the water. Humus (rotting leaves) is another source of phenol that may leach into spring water.
Other natural sources of phenol include:
- Tea
- Vanillin (found in vanilla as well as synthetic vanilla)
- Smoke (including smoked meats)
- Salicylate-containing foods
- Whisky (at least some whisky, e.g. Islay scotch whisky)
- Wine
Phenol Toxicity
Although tolerated in small does, large does are quite toxic.
From Wikipedia:
Phenol and its vapor are corrosive to the eyes, the skin, and the respiratory tract. Repeated or prolonged skin contact with phenol may cause dermatitis, or even second and third-degree burns due to phenol’s caustic and defatting properties. Inhalation of phenol vapor may cause lung edema. The substance may cause harmful effects on the central nervous system and heart, resulting in dysrhythmia, seizures, and coma. The kidneys may be affected as well. Exposure may result in death and the effects may be delayed. Long-term or repeated exposure of the substance may have harmful effects on the liver and kidneys.” There is no evidence to believe that phenol causes cancer in humans. Besides its hydrophobic effects, another mechanism for the toxicity of phenol may be the formation of phenoxyl radicals.
Chemical burns from skin exposures can be decontaminated by washing with polyethylene glycol, isopropyl alcohol, or perhaps even copious amounts of water. Removal of contaminated clothing is required, as well as immediate hospital treatment for large splashes. This is particularly important if the phenol is mixed with chloroform (a commonly-used mixture in molecular biology for DNA & RNA purification from proteins).
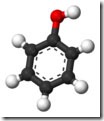
Phenol Chemical Structure
![]() The chemical formula for phenol is C6H5OH.
The chemical formula for phenol is C6H5OH.
At its core is a benzene ring, with at least one hydroxyl group attached.
Salicylate and Salicylic Acid
Salicylate is a salt or ester of salicylic acid. Salicylic acid is made from phenol. It is used to make aspirin and also as a food preservative.
{ 13 comments… read them below or add one }
← Previous Comments